

RECLAMACION: “Coronavirus horror: Volunteer in Oxford University’s COVID-19 vaccine trial DIES”
REVIEW
News that a volunteer in the AstraZeneca COVID-19 vaccine trial died was announced by the Brazilian Health Regulatory Agency (ANVISA) on 21 October 2020. A review by an independent committee that oversees the trial’s safety concluded that there were no safety concerns regarding the trial. As a result, ANVISA allowed the trial to continue, according to reports by the BBC and Reuters. BBC and Bloomberg also reported that the volunteer did not receive the COVID-19 vaccine.
However, other outlets that reported the incident, such as the U.K. tabloid The Daily Express and Health Nut News, did not clarify in their articles that the deceased volunteer had not received the COVID-19 vaccine, nor did they explain that the committee which reviewed the trial found no safety concerns. Health Nut News was also reported as part of a list of websites that have a history of publishing vaccine misinformation.
The incomplete information presented to readers in these articles has stoked fears about the COVID-19 vaccine and anti-vaccine sentiment, as can be seen in shares of the articles here and here. Indeed, both articles went viral on Facebook, receiving a total of more than 95,000 interactions on Facebook at the time of this review’s publication, according to CrowdTangle, a social media analytics tool. By contrast, the articles from Bloomberg and Forbes, which make it clear that the incident is unrelated to the vaccine, only received a total of approximately 11,000 interactions on Facebook.
This is not the first time that misinformation about this vaccine has gone viral. Health Feedback previously reviewed an article published in April 2020 falsely claiming that a volunteer who received the vaccine had died and another article published in May 2020 inaccurately claiming that the vaccine is ineffective against COVID-19.
The vaccine developed by Oxford and AstraZeneca and used in the trial is based on a virus that was modified to contain genetic material encoding the spike protein of the novel coronavirus (SARS-CoV-2). The spike protein enables the virus to enter human cells and establish infections. The modified virus, which is known as a viral vector, which is a chimpanzee adenovirus vaccine vector (ChAdOx1), was developed at Oxford’s Jenner Institute and chosen for vaccine development due to its ability to induce a strong immune response. In addition, the vector cannot replicate in humans and therefore cannot cause infections, making vaccines with this vector safer for people.
When injected, the vaccine induces the body to produce the SARS-CoV-2 spike protein, which then triggers an immune response, including antibody production, against these proteins. If the body encounters the SARS-CoV-2 virus later, it can quickly mount a defense against the virus due to previous exposure to the spike protein, thereby protecting against future infection. (Learn more about the vaccine.)
Vaccine testing to ensure safety and efficacy takes place in stages. The figure below from the U.S. Centers for Disease Control and Prevention explains each phase of the clinical trials involved in vaccine development.
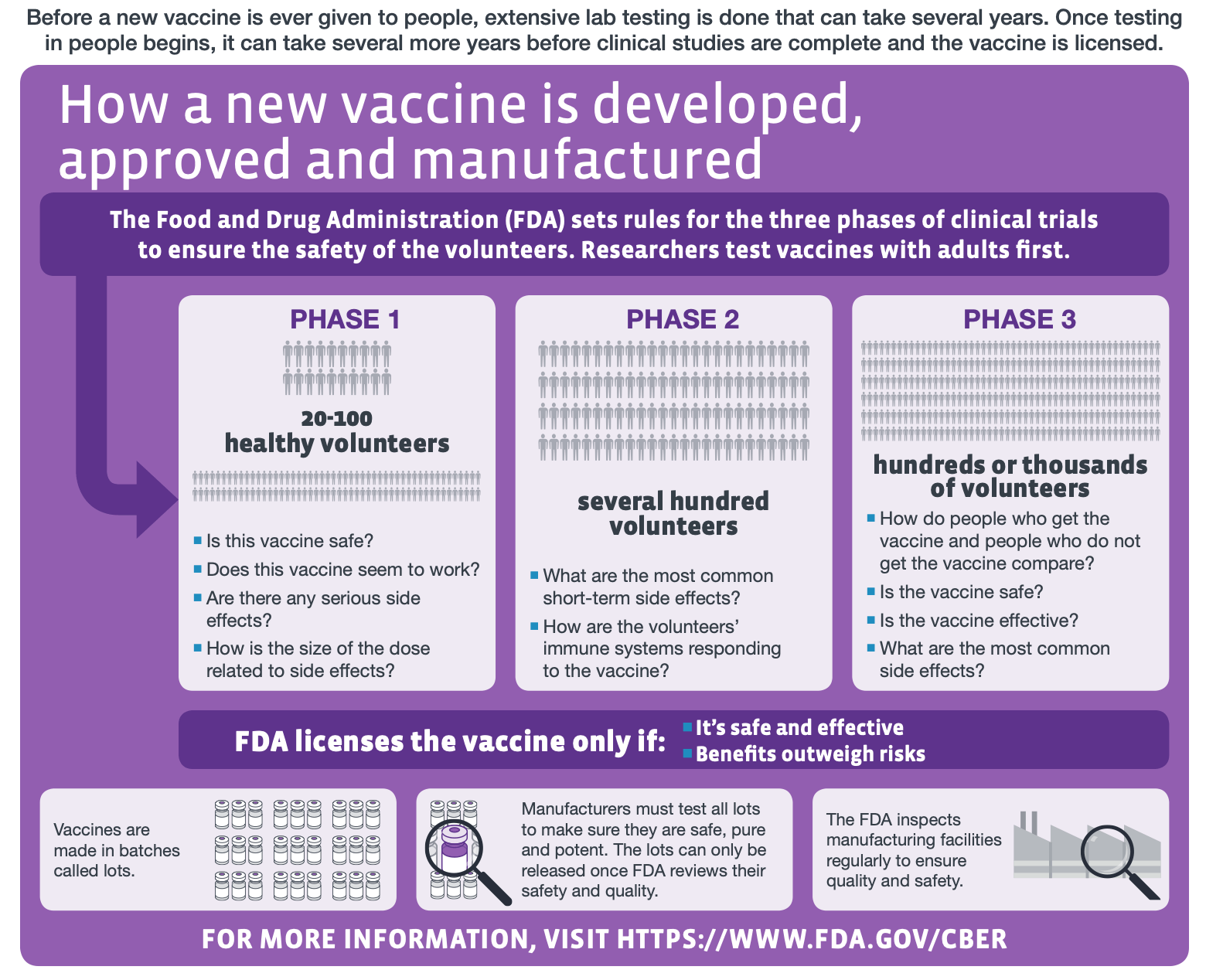
Figure—The different stages of human clinical trials in vaccine development (modified from the original graphic by the U.S. Centers for Disease Control and Prevention).
According to this Oxford University website, the AstraZeneca-Oxford vaccine trial underwent Phase I and Phase II with more than 1,000 participants in the U.K., which primarily assess vaccine safety. The trial progressed to Phases II and III in the U.K., and Phase III studies were also conducted in other countries, including Brazil, South Africa, and the U.S. The Phase III studies, which determine the vaccine’s efficacy, divides volunteers into two groups, one of which receives the COVID-19 vaccine and the other receives an existing licensed vaccine for meningitis named MenACWY.
UPDATE (30 Oct. 2020):
After our evaluation was published, The Daily Express corrected their article (see archive) to provide additional context for readers, by clarifying that the volunteer who had died had not received the COVID-19 vaccine.


