
Misleading: The article wrongly claimed that the absolute risk reduction (ARR) from the COVID-19 vaccines indicates only “minuscule” benefit. While ARR can be a helpful measure of vaccine efficacy, interpreting it correctly requires an understanding of how it is influenced by a study’s design. It is also calculated differently from relative risk reduction, hence its relatively lower value. Real-world evidence shows that the RNA vaccines are highly effective at preventing illness, and death.

RECLAMACION: 'Biodistribution of lipid nanoparticles which carry the mRNA show that the ovaries get the highest concentration. This turns the ovaries into a very large manufacturing plant to turn out toxic spike protein'; the benefit of the vaccine is “minuscule“, with “less than a .5% reduction in absolute risk“
REVIEW
An article published by Steve Kirsch on TrialSite News in late May 2021 claimed that RNA vaccines would affect fertility. Kirsch is the CEO of a digital money platform. According to social media analytics tool CrowdTangle, the article received more than 14,000 interactions on Facebook to date, including more than 5,000 shares. The article also received more than 1,800 interactions on Reddit, primarily in communities focused on conspiracy theories and COVID-19 skepticism.
The narrative that vaccines affect fertility isn’t unique to COVID-19 vaccines. Claims feeding this narrative have been propagated for years by anti-vaccine groups, taking on different forms over time—even though scientific evidence doesn’t support these claims, as shown in earlier reviews by Health Feedback on the flu vaccine and the human papillomavirus vaccine, and this fact-check on the tetanus vaccine by Africa Check.
In the latest iteration of this claim, the TrialSite News article and others presented information from official documents submitted by Pfizer to Japan’s regulator Pharmaceuticals and Medical Devices Agency (PMDA) as evidence for its claim. The article also contains a list of other claims, including several that were debunked previously (see our earlier reviews related to Geert Vanden Bossche, the Vaccine Adverse Events Reporting System, the Cleveland Study by Shrestha et al., comparison to the flu vaccine, and miscarriages in pregnant women). This technique, in which a long list of inaccurate and/or misleading information is propagated repeatedly, is also known as firehosing.
This review primarily focuses on the claims about the Pfizer biodistribution study, spike protein toxicity, and the RNA vaccine efficacy. These come together in the article to ultimately paint a misleading picture of COVID-19 vaccine safety for readers.
The data showed that the injection site retained the highest concentration of lipid nanoparticles over time, not the ovaries
The article’s claim about infertility stems from the misinterpretation that biodistribution data of lipid nanoparticles showed the ovaries to receive the highest amount among all organs and tissues. Lipid nanoparticles enclose the RNA that encodes for the SARS-CoV-2 spike protein and enable cells to take up the RNA. We can assess the veracity of this claim by examining the biodistribution data in the technical documents submitted by Pfizer to Japan’s PMDA (see pages 5 and 6).
This data was obtained by injecting rats with a mix of lipid nanoparticles, which are identical to the ones used in the COVID-19 RNA vaccines, that carry a radioactive “label” (deuterium). Researchers then then measured the level of radioactivity in tissues at different time points after injection. The level of radioactivity acts as a proxy measurement for how much lipid nanoparticle is present in a given tissue. Changes in the level over time provide scientists with an idea of how long it takes for the body to eliminate the particles.
The article’s interpretation of the biodistribution data is inaccurate. As Abraham Al-Ahmad, an associate professor in pharmacology at Texas Tech University, pointed out in a blog post, the data showed that the injection site had the greatest accumulation of lipid nanoparticles, followed by the liver. Specifically, the peak concentration at the injection site was 52.6% of the administered dose at one hour post-injection. That of the liver was 18.1% of the administered dose at eight hours post-injection (see Table 1). A microgram is one-millionth of a gram.
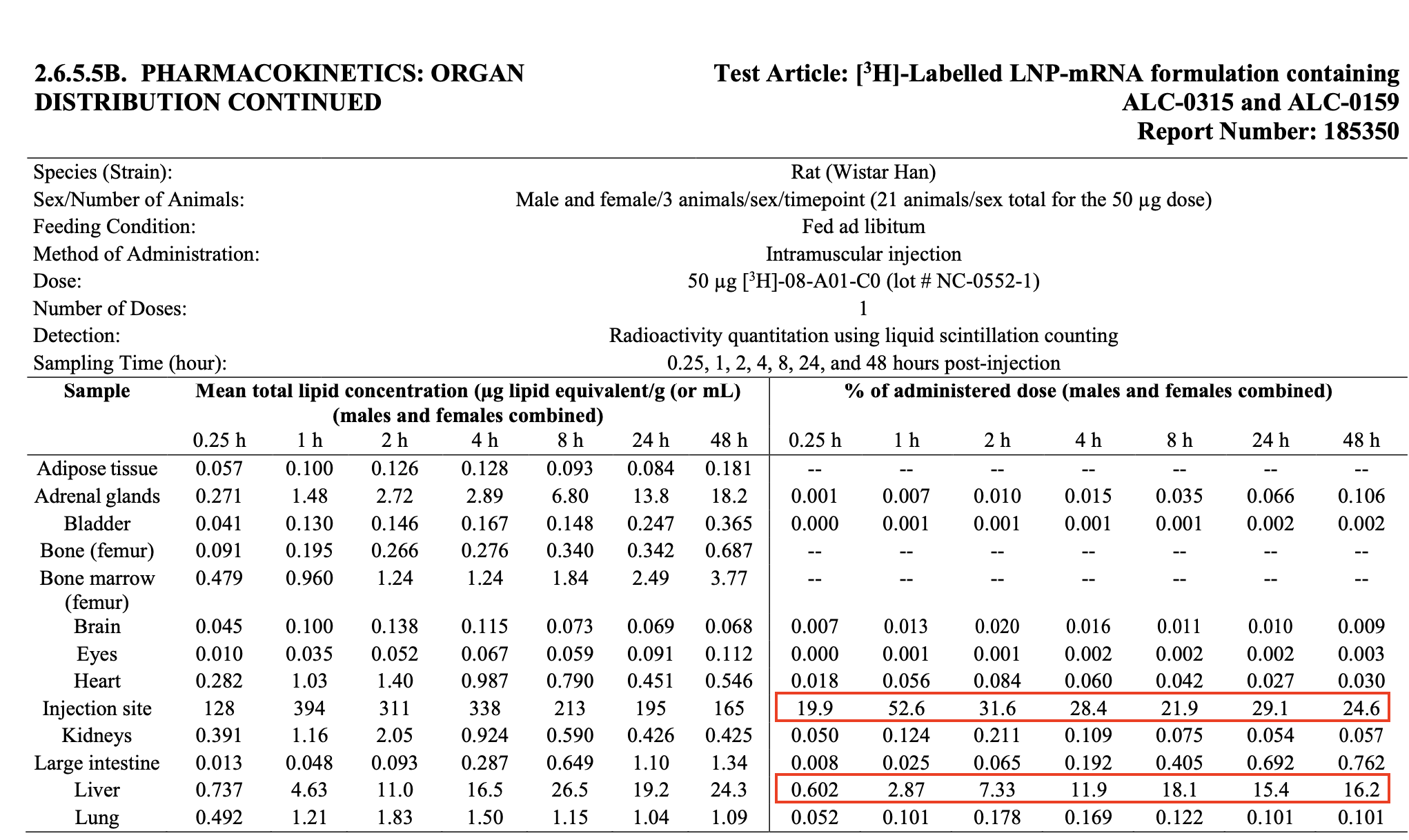
Table 1. The amount of lipid nanoparticles detected in organs and tissues of rats at various intervals up to 48 hours. The rats were injected with 50 micrograms of lipid nanoparticles via the intramuscular route. Observe that the injection site retained more than half the administered dose at 1 hour post-injection and showed the highest concentration relative to other organs and tissues, regardless of the time point.
However, instances of this claim, as seen in the TrialSite News article, tend to omit the table containing the data for the liver and injection site, instead drawing attention only to the data for the ovaries.
The peak concentration in the ovaries, occurring at 48 hours post-injection, was just 0.095% of the administered dose (see Table 2).
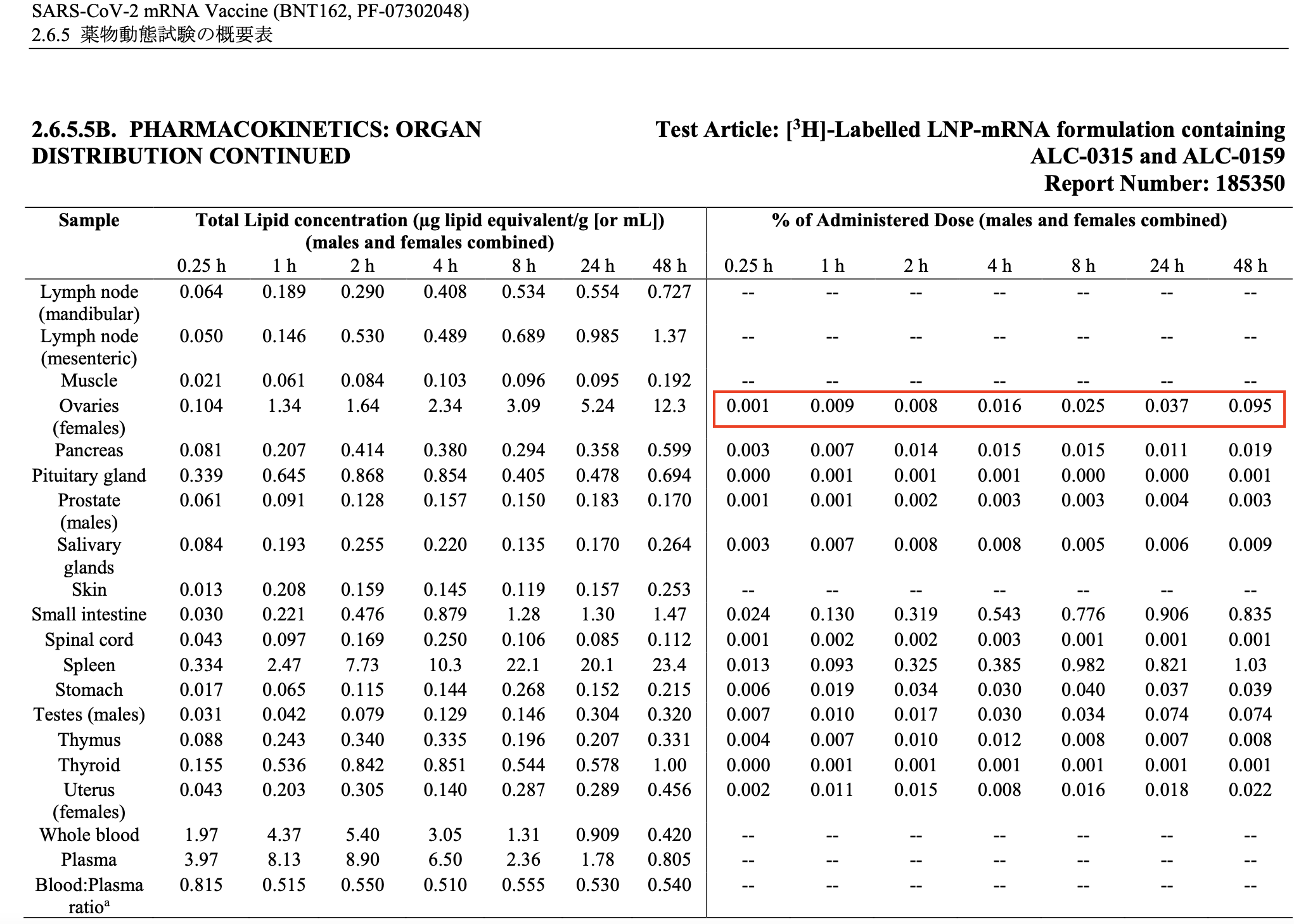
Table 2. The amount of lipid nanoparticles detected in organs and tissues of rats at various intervals up to 48 hours. In the original document submitted by Pfizer, this table followed that shown in Table 1.
Apart from the inaccurate interpretation of data, another critical aspect of the biodistribution experiment that TrialSite News failed to consider is the amount of lipid nanoparticles administered in the rats and its relevance, or more precisely, its lack thereof to the amount present in RNA vaccines given to people.
The study administered 50 micrograms of lipid nanoparticles to each rat. As explained by David Gorski, a professor of surgery at Wayne State University and editor of Science Based Medicine, this would effectively translate to a much higher dose in rats than in humans. This is due to the large difference in body weight:
“The human vaccine contains […] basically ~0.46 mg lipids or 460 μg. Let’s just round it up to 500 μg (0.5 mg). That’s approximately 10x the dose given to the rats. However, for the typical ‘70 kg’ male, 0.5 mg represents a per-weight dose of 0.0071 mg/kg, or 7.1 μg/kg. Let’s compare to the rats, which generally weigh around 200 g (0.2 kg), give or take, at 8 weeks, which is the usual age rodents are used for experiments. That would translate to a per-weight dose of ~250 μg/kg. Even if you used much older rats, who can weigh as much as twice as much, that would still translate to a dose of 125 μg/kg. So we’re looking at a lipid nanoparticle [dose] of ~18-35 times higher (as a rough estimate) than the typical adult human dose.”
In other words, the dose administered to rats was far higher than the dose used in people.
There isn’t evidence showing that COVID-19 RNA vaccines are causing fertility problems. Notably, some participants in the clinical trials for COVID-19 RNA vaccines became pregnant during the trial. The Vaccines and Related Biological Products Advisory Committee that reviews the safety, effectiveness, and appropriate use of vaccines published briefing documents that detailed the outcomes in pregnant trial participants.
For the Pfizer-BioNTech vaccine, the briefing document stated:
“Twenty-three pregnancies were reported through the data cut-off date of November 14, 2020 (12 vaccine, 11 placebo). […] Unsolicited [adverse events] related to pregnancy include spontaneous abortion and retained products of conception, both in the placebo group.”
For the Moderna vaccine, the briefing document stated:
“Thirteen pregnancies were reported through December 2, 2020 (6 vaccine, 7 placebo). […] Unsolicited [adverse events] related to pregnancy include a case of spontaneous abortion and a case of elective abortion, both in the placebo group.”
As the above shows, adverse events related to pregnancy for both trials occurred only in the group that didn’t receive the RNA vaccine. That being said, these numbers are too small to provide any meaningful information about the safety of the COVID-19 vaccines in pregnant women. For this reason, researchers are conducting clinical trials specifically to address the question of the vaccines’ safety and efficacy in pregnant women.
Since pregnant women are more likely to develop severe COVID-19 and complications and the existing data doesn’t indicate that safety concerns in pregnant women are likely, both the U.S. Centers for Disease Control and Prevention as well as the American College of Obstetricians and Gynecologists state that pregnant women should be given access to COVID-19 vaccines, if they wish to be vaccinated.
The amount of spike protein generated by vaccination is extremely small; scientific evidence shows it doesn’t cause harm
The article also cited Canadian immunologist Byram Bridle, whose claims about the purported dangers of spike proteins induced by RNA vaccines went viral in early June 2021. It is of note that Bridle’s academic profile on the University of Guelph website (archived here) showed that his laboratory is doing work on COVID-19 vaccine development, albeit based on a different technology from RNA. Health Feedback covered Bridle’s claims in an earlier review; scientists who examined the claim found it to be misleading.
Bridle misinterpreted findings from a study in hamsters[1] and a study in vaccinated people[2]. In the animal study, which was conducted at the Salk Institute, hamsters that were infected with pseudovirus carrying the SARS-CoV-2 spike protein showed signs of damage in the blood vessels. Researchers concluded from their findings that vaccination could protect against blood vessel damage—directly contradicting Bridle’s claim that the study supports his claim.
In the other study, Ogata et al. measured the amount of spike protein in the blood of 13 people who received the Moderna COVID-19 vaccine. Researchers detected peak levels of spike protein up to 6812 picograms per milliliter during the first week after the first dose. A picogram is one thousand billionth of a gram. And after the second vaccine dose, spike protein was no longer detectable in the blood of these people. The study didn’t report any detrimental effects.
On Twitter, Uri Manor, one of the senior authors of the Salk Institute study, highlighted that the levels detected by Ogata et al. was about 100,000 times less than the amount used in their study:
Congrats to @OgataAlana on this important study. Many asked how much spike protein gets into circulation after vaccination. Turns out to average ~30-40 pg/mL for a few days then disappears.
FYI: This is ~100,000x less than used in our paper (4 ug/mL).https://t.co/k5AChClhI5 pic.twitter.com/xgzJ4RerYG
— Uri Manor (@manorlaboratory) May 21, 2021
In summary, neither of the two studies Bridle cited support his claim, and the first directly contradicts it. Clinical trials and safety monitoring of ongoing vaccination campaigns show that the RNA vaccines are very safe and their benefits outweigh their risks.
Clinical trial data showed that COVID-19 vaccines significantly reduce the risk of severe disease and death; the article misused the figure for absolute risk reduction
The TrialSite News article claimed that the vaccine benefits were “minuscule”, citing “less than a .5% reduction in absolute risk”. Similar claims also appeared in social media posts claiming that the absolute risk reduction (ARR) shows the RNA vaccines are ineffective and that the figure of 95% reported by the media is deceptive (see example), citing a Lancet Microbe comment[3].
These claims are inaccurate and misleading. Piero Olliaro, one of the authors of the Lancet comment, told Lead Stories that their comment had been grossly misinterpreted:
“It is extremely disappointing to see how information can be twisted and how divisive discussions have become especially on COVID-19 vaccines, as they obviously overlap with general vaccine hesitancy and antivax segments of the population.
Bottom line: these vaccines are good public health interventions. Importantly, the effects of vaccination should not be seen merely as reducing individual risk, but its effects on reducing the risk in the entire population, with all its ramifications – reducing stress to health systems, hospital bed occupancy, societal costs, effects on economy, etc… a long list.
We do not say vaccines do not work. We say vaccines do work, and add considerations about intrinsic vaccine efficacy and their effectiveness when used in different populations.”
Olliaro also clarified their message in the Lancet Microbe comment, which was intended to highlight the fact that comparing relative risk reduction (RRR) between different vaccines can be misleading due to differences between populations, that even vaccines with a relatively lower RRR can still be effective in the real world, and that both ARR and RRR should be reported together to provide readers with a more accurate and comprehensive idea of vaccine efficacy.
In Olliaro’s own words:
“It is incorrect to compare [vaccines] based on clinical trials conducted in different conditions, using relative risk reduction (RRR calculated as explained in the paper), and assume vaccines with lower RRR do not work well enough. Studies should therefore report also absolute risk reduction (ARR, calculated as explained in the paper).
Why? The RRR is a measure of vaccine efficacy in preventing disease in those at risk of getting infected and becoming ill; the ARR is a measure of vaccine effectiveness in a population where a certain proportion of individuals will get diseased without vaccine. ARR is more difficult to understand, but can be conveniently translated in NNV (number need to vaccinate = how many people need to be vaccinated in a population with a given background risk of getting covid-19 without vaccine to prevent one more case of covid-19.)
The article explains these concepts with numerical examples from vaccine trials, and that even vaccines with relatively lower RRR are totally worthy.
These numbers are evidence of effectiveness, since even the least ‘effective’ vaccine (AstraZeneca and J&J with RRR of ~67%) you just need to vaccinate ~80 people to prevent one case in populations where just under 2% of people get covid-19 within a certain period of time. This also shows that 2 vaccines with very similar, high efficacy (RRR ~95%) can have different effectiveness depending again on the risk of covid-19: NNV for Moderna vaccine ~80, Pfizer ~120 (the latter was studied in the population with the lowest risk of all).”
Experts at the Meedan Health Desk also explained that the approach used in the clinical trials means that the ARR “will always appear low”:
“Researchers looked at vaccine efficacy once a specific number of COVID-19 cases had been confirmed in vaccine trials. With this approach, the ARR will always appear low, because it is tied to the number of cases confirmed in the study. Let’s say a study enrolled 20,000 patients into the control group and 20,000 in the vaccine group. In that study, 200 people in the control group got sick and 0 people in the vaccine group got sick. Even though the vaccine efficacy would be a whopping 100%, the ARR would show that vaccines reduce absolute risk by just 1% (200/20,000 = 1%). For the ARR to increase to 20% in our example study with a vaccine with 100% efficacy, 4,000 of the 20,000 people in the control group would have to get sick (4,000/20,000 = 20%). From a public health perspective, we would not want to wait for more control group infections and a higher ARR before sharing the study findings.”
Health Desk also raised other caveats about the ARR:
“It is important to know a few things about absolute risk reduction (ARR) and vaccine trials:
1. How much a virus is spreading, as well as measures that people take to prevent getting sick (e.g., wearing masks, social distancing), can influence what absolute risk reduction looks like.
2. Clinical trials are relatively short term. Using an ARR for trial data can make a vaccine seem less promising than the effect that a vaccine would have on a person’s risk over a longer period of time.”
In summary, both the RRR and ARR provide useful information about the efficacy of a vaccine or any other medical intervention. But the article misrepresented the ARR as evidence that the vaccine doesn’t work, which isn’t true.
Evidence in the real world demonstrates that the COVID-19 vaccines are effective. A study in Israel on more than 590,000 healthcare workers showed that the Pfizer-BioNTech COVID-19 vaccine was more than 90% effective in preventing symptomatic COVID-19, hospitalization, and death[4], one week after the second dose.
In addition, both the U.S. and the U.K. observed that unvaccinated people are making up the majority of COVID-19 cases requiring hospitalization, suggesting that vaccinated people are less likely to develop serious illness requiring medical care as compared to unvaccinated people.
Conclusion
Overall, the TrialSite News article contains several inaccurate and misleading claims. It is false to claim that Pfizer’s biodistribution study showed lipid nanoparticles accumulating in the highest concentration in the ovaries. Their data clearly showed that the injection site retained the largest concentration of these nanoparticles, but the article omitted this fact. The evidence so far doesn’t show that COVID-19 vaccination affects ovaries, leads to a higher risk of adverse events during pregnancy, or affects fertility.
The claim by Byram Bridle that spike protein generated by vaccination is toxic is based on misinterpretations of two studies, neither of which actually reported detrimental effects from vaccination. One study examined spike protein’s effect during infection in animals, while the other reported transient, infinitesimal amounts of spike protein in the blood of vaccinated individuals, with no ill effects.
While ARR provides helpful information on vaccine efficacy, it also needs to be interpreted in the context of the study’s design. The relatively smaller ARR compared to the RRR isn’t proof that the vaccines are ineffective, but reflects the difference in the way it is calculated. It is also influenced by other factors such as the non-pharmaceutical measures used to mitigate the spread of COVID-19. Real-world evidence shows that the RNA vaccines are highly effective at preventing illness and death.
REFERENCES
- 1 – Lei et al. (2021) SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circulation Research.
- 2 – Ogata et al. (2021) Circulating SARS-CoV-2 Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clinical Infectious Diseases.
- 3 – Olliaro et al. (2021) COVID-19 vaccine efficacy and effectiveness—the elephant (not) in the room. The Lancet Microbe.
- 4 – Dagan et al. (2021) BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. New England Journal of Medicine.


