- Health
Saliva might be an accurate, less-invasive alternative to nasopharyngeal swabs for COVID-19 diagnosis; more research is needed to standardize its use in molecular testing
Key takeaway
Due to its reliability and relative ease of specimen collection, the nasopharyngeal swab is considered the gold-standard procedure for collecting cells and secretions from the back of the throat for COVID-19 diagnostic testing. In contrast, the accuracy of less invasive testing of alternative specimens, such as saliva, is still under evaluation. Several laboratories have developed at-home kits for the collection of nasal mucus and saliva samples that are then shipped to a laboratory for analysis. However, specimen collection is a key step in clinical diagnosis, and experts have raised concerns about how potential flaws in at-home sample collection may impact the accuracy of these tests.
Reviewed content
Verdict:
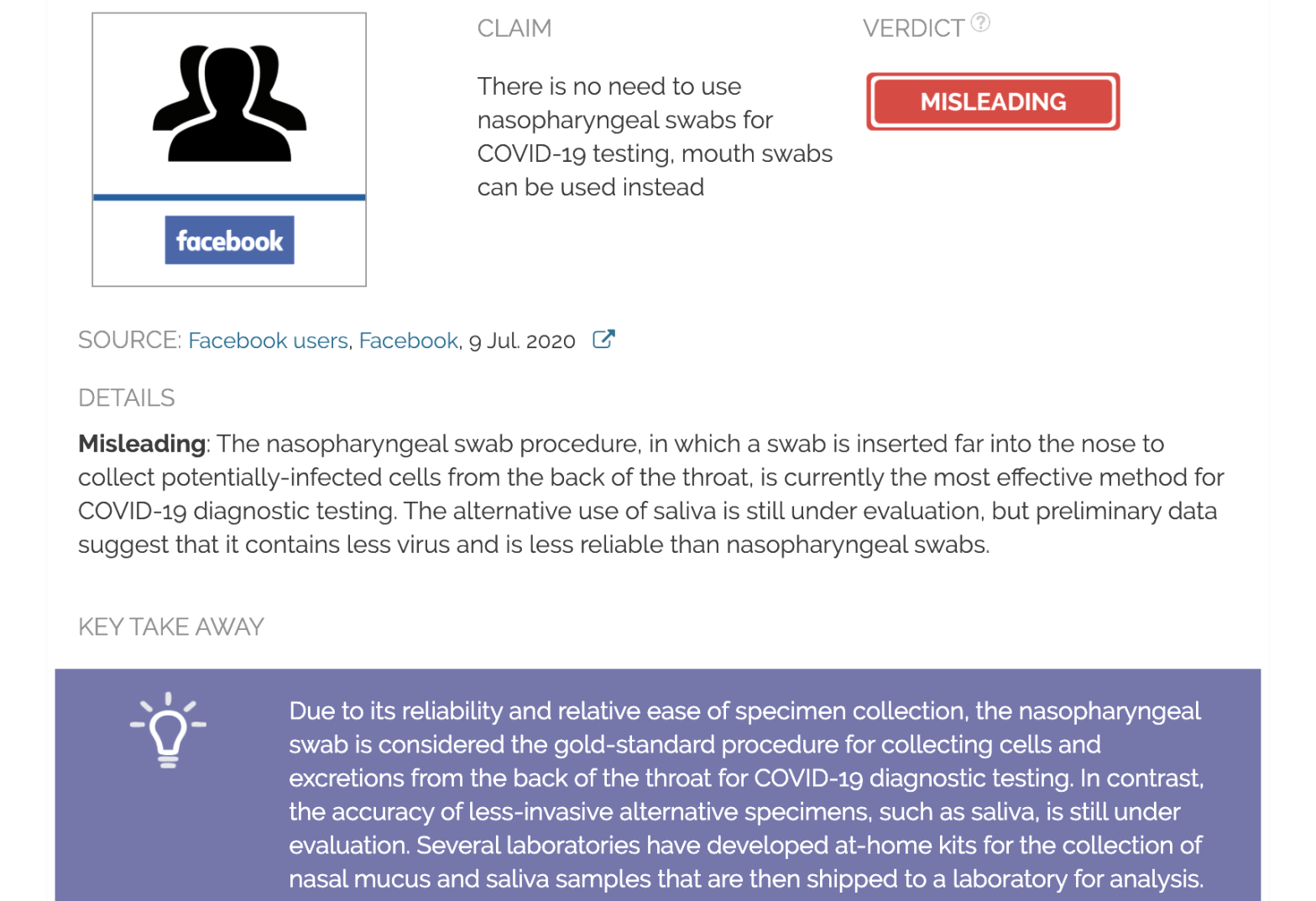
Claim:
There is no need to use nasopharyngeal swabs for COVID-19 testing, mouth swabs can be used instead
Verdict detail
Misleading: The nasopharyngeal swab procedure, in which a swab is inserted far into the nose to collect potentially infected cells from the back of the throat, is currently the gold-standard method for COVID-19 diagnostic testing. Recent evidence suggests that saliva samples might be as accurate as nasopharyngeal swabs for COVID-19 molecular diagnosis. However, the alternative testing of saliva is still under evaluation.
Full Claim
“Modern Science can take a swab from the inside of your cheek and do a complete DNA mapping… Why not swab the inside of your highly contagious mouth?”
Review
This 8 July 2020 Facebook post suggests that at-home testing for COVID-19 using mouth swabs, such as those used to collect DNA samples, could be just as effective as the nasopharyngeal swab method. Earlier versions of the claim questioned the need for nasopharyngeal sample collection in which “they have to stick a Q-tip through your nose to the back of your head to swab a sample of COVID” when it would be much easier to collect saliva samples because “a droplet of saliva will infect a whole village at one time”. Similar posts have been circulating widely on Facebook since the beginning of July 2020 and have received more than 90,000 interactions.
The post reflects a misunderstanding of how COVID-19 transmission and testing work. Interim Guidelines from the U.S. Centers for Disease Control and Prevention (CDC) for COVID-19 testing states that “proper collection of specimens is the most important step in the laboratory diagnosis of infectious diseases”. The distribution and quantity (viral load) of the SARS-CoV-2 virus across the respiratory tract depends on the patient and the type of specimen1. Because of the accuracy of the tests using nasopharyngeal specimens, this is considered the gold-standard collection method for COVID-19 diagnostic testing. The procedure involves inserting a long swab into each of the nostrils and through the nasal cavity until it makes contact with the upper part of the nasopharynx. The swab is then rotated to collect potentially infected cells and secretions for testing by reverse transcription polymerase chain reaction (RT-PCR)2.
The post is correct in affirming that mouth swabs are a highly effective collection method for DNA testing. However, such swabs are used to collect cheek cells, not saliva as suggested. Saliva collection does not require a swab—patients are simply asked to spit into a tube. This method is easier to perform and less uncomfortable than the nasopharyngeal swab and is therefore being evaluated as an alternative approach to nasopharyngeal swabs. This method could also reduce the risk of infection for healthcare workers and the need for personal protective equipment and swabs since patients can “collect” the samples themselves.
However, several studies, including a meta-analysis (joint analysis of data from several independent studies), suggest that the viral load in saliva is lower than in nasopharyngeal samples and highly variable between patients3-6. Furthermore, the virus is detected only during the early phase of the infection and then declines rapidly over time7. Hence, saliva testing may be less accurate and could miss a proportion of COVID-19 cases compared to nasopharyngeal testing.
Even though saliva is not a preferred specimen in COVID-19 diagnostic testing, it may be complementary to nasopharyngeal swab testing and increase the chances of accurately diagnosing infections. In April 2020, the U.S. Food and Drug Administration (FDA) authorized the emergency use of the first at-home collection kit for COVID-19 testing using nasal swabs. Since then, the FDA has approved several other at-home collection devices for nasal mucous samples (Everlywell and KPMAS kits) and saliva samples (Rutgers, Phosphorus, and LetsGetChecked kits). While experts recognize the merits of these kits in terms of comfort and safety, they have also raised serious concerns about their reliability due to potential errors in at-home sample collection.
In a letter to FDA Commissioner Stephen Hahn, the American Association for Clinical Chemistry (AACC) recommended that the FDA issue a warning about the accuracy of COVID-19 at-home tests. The AACC is a global scientific and medical organization providing insight and guidance in the field of clinical laboratory science and its application to healthcare. According to the AACC, “problems commonly occur with self-collection that can affect the quality of the sample and, therefore, the subsequent test result”. Major concerns involve sample quantity, sterility, incorrect handling, or viral integrity during shipping. False-negative results could impact public health since infected individuals may “fail to be treated properly and take appropriate actions to prevent the spread of the disease”.
The claim that “a droplet of saliva will infect a whole village at one time” is inaccurate. Although the specific infective capacity of saliva and its role in transmission are not clear yet8, this claim overestimates the infectivity of a single drop. The highest risk of contagion has primarily been reported for people sharing a confined space for long periods of time and releasing thousands of respiratory droplets9. As previously explained by Health Feedback, respiratory droplets are small particles of mucus or saliva that a person releases when coughing, sneezing, or talking and are considered the main mode of COVID-19 transmission.
In contrast, droplets smaller than 5 micrometers in diameter can form aerosols which float farther away than respiratory droplets, becoming an alternative transmission pathway under certain circumstances. On 6 July, 250 scientists issued a letter calling on the World Health Organization (WHO) to consider airborne transmission as a significant mode of disease transmission for COVID-19. This led the WHO to acknowledge “the possibility of aerosol transmission, combined with droplet transmission, particularly in indoor settings with poor ventilation”.
A 17 November 2020 study by Babady et al. published in The Journal of Molecular Diagnostics found that the diagnostic accuracy of saliva samples for PCR testing was similar to that of nasopharyngeal swabs[10]. The agreement between saliva and nasopharyngeal swabs was 97.7%, with a sensitivity of 94.1%. Later research, including three meta-analyses from previous studies, further supported the use of saliva as an alternative sample for PCR screening and diagnosing of COVID-19[11-13], particularly in people with mild or no symptoms[14].
The use of saliva instead of nasopharyngeal swabs would potentially allow large-scale testing by facilitating sample collection without the need for trained staff and reducing the risk of exposure. However, available data is limited because many of the studies published so far were relatively small and used different methodologies or populations[15]. Factors such as an adequate sample collection, disease severity and timing, or the type of PCR kit used may influence the accuracy of saliva-based PCR tests[16]. The development of standard protocols will allow researchers to assess the reliability of saliva specimens for COVID-19 diagnosis.
[Scientific research on COVID-19 is ongoing. This review will be updated as new information becomes available.]
This review was updated with new studies evaluating the accuracy of saliva compared to nasopharyngeal swabs for PCR testing[10-16]. The results from these studies show that saliva might be an accurate alternative sample for PCR screening and diagnosing of COVID-19, particularly in asymptomatic or mildly-symptomatic people. However, collection and processing methods for saliva are largely unstandardised, and therefore, nasopharyngeal samples remain the gold standard for COVID-19 PCR and rapid diagnostic testing.
REFERENCES
- 1 – Yang et al. (2020) Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. MedRxiv. [Note: This is a pre-print that has not yet been peer reviewed or been published in a journal at the time of this review’s publication.]
- 2 – Marty et al. (2020) How to Obtain Nasopharyngeal Swab Specimen. New England Journal of Medicine.
- 3 – Wang et al. (2020) Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA.
- 4 – Czumbel et al. (2020) Saliva as a Candidate for COVID-19 Diagnostic Testing: A Meta-Analysis. Frontiers in Medicine.
- 5 – Wölfel et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature.
- 6 – Williams et al. (2020) Saliva as a non-invasive specimen for detection of SARS-CoV-2. Journal of Clinical Microbiology.
- 7 – Griesemer et al. (2021) Evaluation of specimen types and saliva stabilization solutions for 2 SARS-CoV-2 testing. Journal of Clinical Microbiology.
- 8 – Xu et al. (2020) Saliva: potential diagnostic value and transmission of 2019-nCoV. International Journal of Oral Science.
- 9 – Ma et al. (2021). Coronavirus Disease 2019 Patients in Earlier Stages Exhaled Millions of Severe Acute Respiratory Syndrome Coronavirus 2 Per Hour. Clinical Infectious Diseases.
- 10 – Babady et al. (2020) Performance of Severe Acute Respiratory Syndrome Coronavirus 2 Real-Time RT-PCR Tests on Oral Rinses and Saliva Samples. The Journal of Molecular Diagnostics.
- 11 – Butler-Laporte et al. (2021) Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2. JAMA International Medicine.
- 12 – Lisboa Bastos et al. (2021) The Sensitivity and Costs of Testing for SARS-CoV-2 Infection With Saliva Versus Nasopharyngeal Swabs. Annals of Internal Medicine.
- 13 – Tsang et al. (2021) Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. Lancet Infectious Diseases.
- 14 – Teo et al. (2021) Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 infection. Scientific Reports.
- 15 – Tan et al. (2021) Saliva as a gold-standard sample for SARS-CoV-2 detection. Lancet Respiratory Medicine.
- 16 – Fernández-González et al. (2021) Performance of Saliva Specimens for the Molecular Detection of SARS-CoV-2 in the Community Setting: Does Sample Collection Method Matter? Journal of Clinical Microbiology.